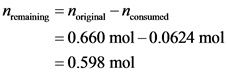After the reaction, how much octane is left?
2 C8H18 + 25 O2 --> 16 CO2 + 18 H20
0.660 mol of octane is allowed to react with .780 mol of oxygen. Oxygen is the limiting reactant.
After the reaction, how much octane is left?
Concepts and reason
This is based on the concept that the limiting reagent decides how much amount of other reactants will be consumed when the reaction takes place. This decides the amount of product formed. Limiting reagent itself consumed fully and when it consumed, the reaction stops. This is only determined by the help of a balanced chemical equation. Limiting reagent also helps us to identify the percentage yield of the reaction.
Fundamentals
Limiting reagent is also called as limiting reactant. In a reaction, it is limiting reagent consumed totally and the remaining reactants are called as excess reagents.
Percentage theoretical yield is defined as the amount of the formation of the product when the limiting reagent is consumed fully.
Answer:
Consider the reaction:
![]()
This reaction is balanced.
Calculation for the amount of ![]() is consumed is as follows:
is consumed is as follows:
![]()
Calculate the remaining moles as follows: